TOP > News Release > Otsuka Pharmaceutical Factory launches "Tumguide LED Light Source" and "Tumguide Fiber" as medical devices that configure "Tumguide," a device for checking the position of the nasogastric tube tip
September 13, 2023
Otsuka Pharmaceutical Factory, Inc. (Head Office: Naruto, Tokushima, Japan; President and Representative Director: Shinichi Ogasawara; "OPF") will launch a general medical device (general-purpose light source) "Tumguide® LED Light Source" and a general medical device (gastroesophageal sterile tube and catheter for temporary use) "Tumguide® Fiber" that configure a medical device for checking the position of the nasogastric tube tip "Tumguide®," on October 17, 2023.
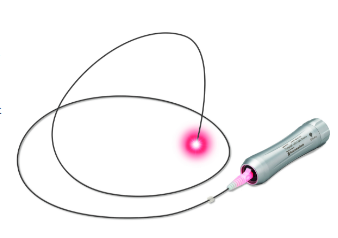
Tumguide is a medical device that allows you to visually check the tip position during nasogastric tube insertion by observing the Biological Transparent Illumination*1 emitted from the optical fiber tip inserted into the nasogastric tube (feeding tube) from outside the body. It was co-developed with a medical device venture company, Neuroceuticals, Inc. (Head Office: Bunkyo-ku, Tokyo, Japan; President and Representative Director: Shinya Miike). The device comprises "Tumguide LED Light Source," which applies original light-gathering technology and adopts red light with high biological transparency to the emitted LED light, and sterile "Tumguide Fiber" with an added detection function aimed for avoiding infection caused by reuse.
For nutritional management in patients who have difficulty eating an oral diet, enteral nutrition, a physiological route of administration, is considered first, and a nasogastric tube, in which a feeding tube is inserted through the nose into the stomach, is often used to administer nutrients. However, medical accidents occur by injecting nutrients without realizing that the tip of the tube has been accidentally inserted into the lung, not the stomach. To prevent such accidents, aspiration of gastric juice, confirmation of the pH of aspirated liquid, confirmation of the tube tip position by taking X-rays, etc., have been made in healthcare settings. Nevertheless, there are still reported cases of incorrect insertion.
Tumguide is a medical device that connects a light source device to an optical fiber inserted into a feeding tube and it is inserted into the stomach through esophagus while the light at the tube tip is shining. This medical device enables to visually check the position of the tube tip from outside the body and is expected to contribute to reducing the physical burden on patients and reducing the workload of healthcare professionals such as reducing medical accidents caused by incorrect insertion into the lung and avoiding radiation exposure.
As a company with strength in clinical nutrition such as IV solution and enteral nutrition products. OPF has been providing various products and useful information for appropriate nutritional management. We will continuously provide these and develop innovative products to contribute to solving issues in medical fields, aiming to be the best partner for patients and healthcare professionals in the clinical nutrition field.
Based on the corporate philosophy of "Otsuka-people creating new products for better health worldwide," the Otsuka Group is dedicated to contributing to the health of people around the world.
*1 A light in the wavelength range which transmits through soft tissues, but not for hard tissues such as bones and cartilages.
Tumguide® LED Light Source
|
Medical device classification |
General medical device |
|
Generic name |
General-purpose light source |
|
Class category |
Class I |
|
Medical device notification number |
13B1X10190000016 |
|
Shape, structure and principle |
<Shape, structure> External dimensions: full length 127.5 mm, outer diameter 34 mm (maximum diameter) Weight: approximately 194 g (includes battery) Composition: Tumguide® LED Light Source (this device) 1 unit Battery 2 pieces (one of which is a spare) Battery charger 1 unit AC adapter 1 unit <Principle> This device is a handy-type high-brightness LED light source. |
|
Purpose of use or effect |
This device generates an intense light for general surgery or medical treatment. The light is transmitted through the optical fiber cable to a connected device for medical treatment. |
|
Directions for use |
Refer to the instruction manual of this device for detailed directions of use. ① Take out the Tumguide Fiber aseptically. ② Insert the Tumguide Fiber to a nasogastric tube (a tube which is used for nutritional administration, or intragastric decompression, gastric juice collection, infusion of drugs, cleaning, or removal, etc., of foreign matters from stomach). Fix the stopper at the position with sufficient space so that the tube tip does not stick out. ③ Turn on this device. After confirming that there is sufficient remaining battery charge, connect the Tumguide Fiber to the fiber socket. ④ Insert into the stomach through the esophagus while the tip of the Tumguide Fiber is shining, and confirm the position of the tip. ⑤ After confirming that the nasogastric tube tip has been placed in the stomach, turn off the power and remove the Tumguide Fiber from the fiber socket. ⑥ Remove the Tumguide Fiber promptly. |
|
Storage and expiration date |
<Storage> · Store away from water. · Storage temperature: -25℃ - 50℃ · Storage humidity: 0% - 95% (No condensation) <Expiration date> 5 years (By self-certification [manufacturer]) |
|
Items related to maintenance and inspection |
· Clean the surface of this device with a cloth etc., soaked with a commercially available surface disinfectant or neutral detergent diluted by water. · Do not spray disinfectant to the device. · Do not allow any liquid, such as disinfectant, to enter the fiber socket. |
|
Suggested retail price (excluding tax)*2 |
JPY 400,000 |
|
Manufactured and distributed by |
Neuroceuticals, Inc. |
|
Marketed by |
Otsuka Pharmaceutical Factory, Inc. |
|
Comarketed by |
Otsuka Pharmaceutical Co., Ltd. |
Tumguide® Fiber
|
Medical device classification |
General medical device |
||||||||||
|
Generic name |
Gastroesophageal sterile tube and catheter for temporary use |
||||||||||
|
Class category |
Class I |
||||||||||
|
Medical device notification number |
13B1X10190000015 |
||||||||||
|
Shape, structure and principle |
<Shape, structure> Raw materials (*) of the part which come in contact with blood and body fluids. Insertion site: plastics, coating agent
<Principle> This product connects to the light source device and is inserted to the body while it is inserted in the nasogastric tube. The tip of the insertion site shines by the light from the light source device, which enables to check the tip position visually from outside the body. |
||||||||||
|
Purpose of use or effect |
Insert this product with the nasogastric tube into esophagus and stomach temporarily. By connecting this product to the light source device, the tip position can be visually checked from outside the body. |
||||||||||
|
Directions for use |
Same as the directions for use of the Tumguide LED Light Source. |
||||||||||
|
Storage and expiration date |
<Storage> · Store away from water. · Store away from direct sunlight, high temperature, and high humidity. · Storage temperature: -10℃ - 45 ℃ · Storage humidity: 0% - 95% (No condensation) <Expiration date> As stated on the product (By self-certification) |
||||||||||
|
Packaging |
10 fibers per box |
||||||||||
|
Suggested retail price (excluding tax)*2 |
JPY 23,000 |
||||||||||
|
Manufactured and distributed by |
Neuroceuticals, Inc. |
||||||||||
|
Marketed by |
Otsuka Pharmaceutical Factory, Inc. |
||||||||||
|
Comarketed by |
Otsuka Pharmaceutical Co., Ltd. |
*2 The price is shown as a reference price.