TOP > News Release > Otsuka Pharmaceutical Factory launches OT-Balloon Catheter, an intermittent urological catheter
July 29, 2022
Otsuka Pharmaceutical Factory, Inc. (Head Office: Naruto, Tokushima, Japan; President and Representative Director: Shinichi Ogasawara; "OPF") will launch an intermittent urological catheter "OT-Balloon Catheter®," a medical device, on August 22, 2022.
This product is a reusable catheter for use during self-catheterization for people who have difficulty urinating spontaneously for various reasons. The catheter is temporarily implanted in the bladder by inflating the balloon at the tip of the catheter during the night or when going out. The catheter can be used according to the patient's lifestyle, as self-catheterization is not necessary while it is in place.
In the nursing care field in Japan, which has entered a super-aging society, the importance of excretion independence has been recognized and the need for excretory care is increasing, as evidenced by the review of the additional fee for excretion support in the 2021 revision of nursing care fees. In the event of residual urine or obstruction due to lower urinary tract obstruction, such as neurogenic bladder or prostatic hyperplasia, a method of clean intermittent catheterization is widely used in which urine accumulated in the urinary bladder is catheterized through the urethral meatus at regular intervals to drain it out of the body, and an intermittent balloon catheter product that is easy to use outside the hospital, such as at home care, has been desired.
The product will be marketed by Otsuka Pharmaceutical Factory under an exclusive sales agreement with the manufacturer and distributor, Otsuka Techno Corporation (Head Office: Naruto, Tokushima, Japan; President and Representative Director: Kanji Tsukii; "Otsuka Techno"). Otsuka Techno is developing medical products and precision products as the pillars of its business by utilizing its advanced molding technologies for medical plastic materials for containers, etc., in order to contribute to society through manufacturing.
The management vision of OPF is to be "The Best Partner in Clinical Nutrition." In addition, we are expanding our business areas to cover the entire healthcare process, such as prevention, diagnosis, treatment, and monitoring. Among these, we have been enhancing our products in the fields of urology and urination, including the marketing of Lilium® α-200, a medical device that measures urinary bladder volume, since 2016, and launching its successor product, Lillium® IP200, in June this year, as well as Actreen, a single-use intermittent urinary catheter, in January. We will continue to develop products that can contribute to excretion care for patients.
Based on the corporate philosophy of "Otsuka-people creating new products for better health worldwide," the Otsuka Group is dedicated to contributing to the health of people around the world.
|
Product name |
OT-Balloon Catheter |
|
Classification |
Instrument & apparatus 51, Suckers, tubes and catheters for infusion or drainage |
|
Medical device classification |
Controlled medical device |
|
Classification |
Class II |
|
Generic name |
Intermittent urological catheter |
|
Packaging |
2 pieces |
|
Expiration date |
2 years |
|
Storage |
1. Store in a clean place, away from direct sunlight, high temperature and high humidity, and dust. 2. After use, store the catheter in a case containing disinfectant solution. |
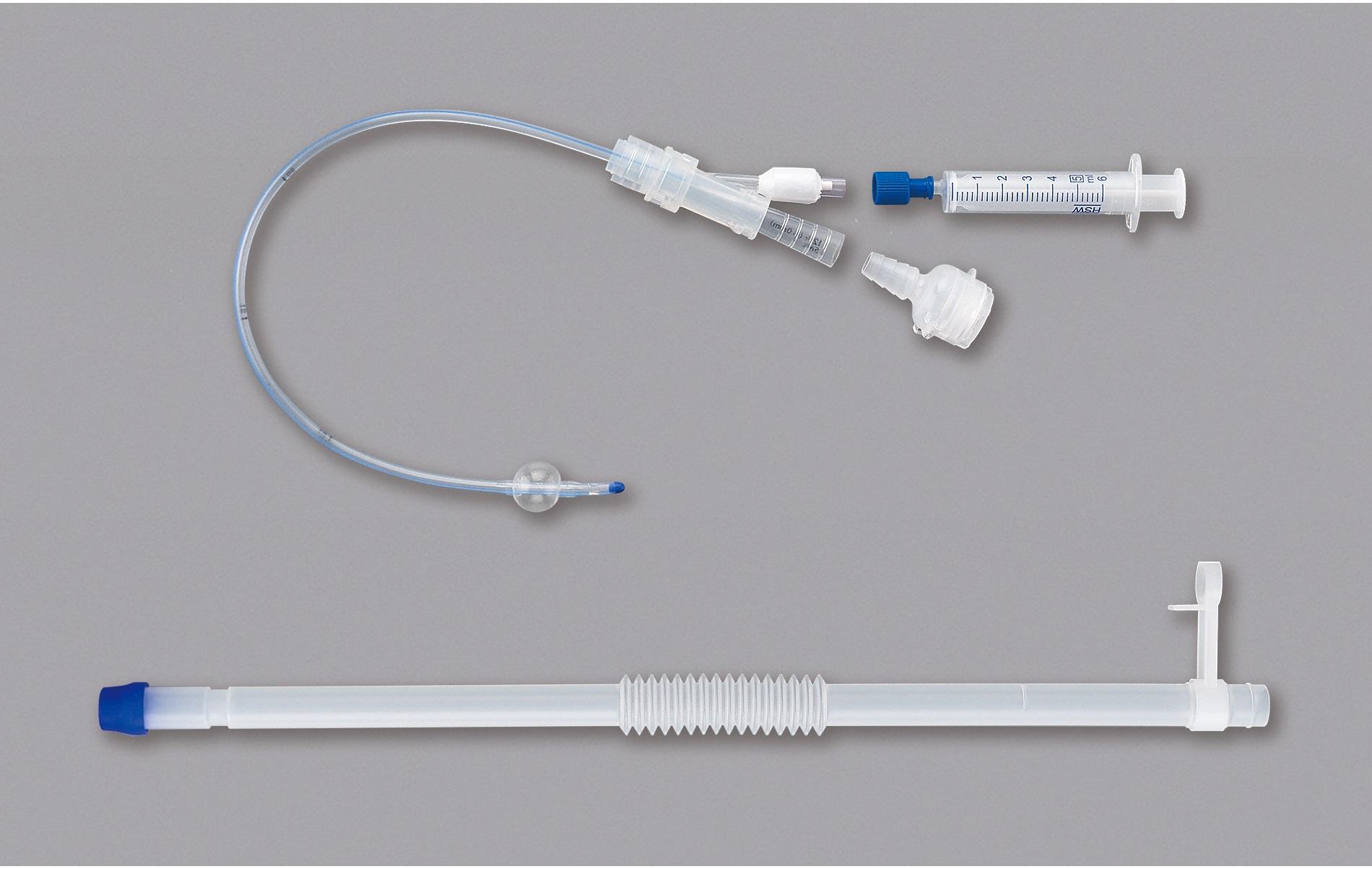
|
Size |
Outer Diameter |
Length |
Balloon Capacity |
|
12 Fr |
4.0 mm |
400 mm |
5 mL |
|
14 Fr |
4.7 mm |
||
|
16 Fr |
5.3 mm |
||
|
Depth marks:50 mm intervals between 100 mm and 200 mm from the tip |
|||
|
Side holes:2 holes |
|||