TOP > Research and Development

The research and development of drugs takes long years and persevering effort. We perform verifications to assure a product’s safety and efficacy, followed by a careful validation of the product’s efficacy through clinical trials.
It takes the constant effort of many people before our pharmaceutical products ever reach actual patients. In this R&D phase, our efforts are always grounded in scientific evidence, and we take the patient’s perspective when evaluating the product’s safety and efficacy. It is therefore crucial to understand conditions in actual healthcare settings; communication with patients and healthcare professionals leads to better research and development.
We also work hard to train our researchers to a high level of technical competence grounded in a wide range of knowledge and tempered by an open, interdisciplinary approach that places priority on corporate ethics. Putting patients first, we will continue improving our R&D capabilities sustainably, provide safe and effective products which accurately assess the needs of patients and healthcare professionals, and keep contributing to people’s health.
In addition to IV solutions, our core product domain and a fundamental pillar of medical treatment, and medical foods, Otsuka Pharmaceutical Factory engages in R&D to develop products which create new value by meeting unmet medical needs in the fields of surgical aid and regenerative medicine products. We see things from the patient’s point of view and develop innovative medical products which are not bound by the constraints of existing concepts, and undertake all these initiatives with enthusiasm and a sense of duty. For example, we are developing a bioengineered pancreatic islet product which could become a major treatment for diabetes patients.
Depending on the circumstances, healthcare professionals often prepare antibiotic solutions or nutrition solutions containing glucose, electrolytes, amino acids, vitamins, trace elements, or all of the above. These solutions are administered intravenously, which is a severe burden for healthcare professionals as it requires an aseptic procedure. We initiated research into the development of a product container that would be both safe and easy to use, which resulted in our Otsuka multi-chamber bag system that enables antibiotics and solvents to be administered in a sterile environment with a single press. Our antibiotic kit solution, the first of its kind in the world, utilizes this technology. More recently we developed the first-ever quad-chamber bag. These products, which help to prevent medication or mixing errors, have been widely acclaimed by medical institutions.

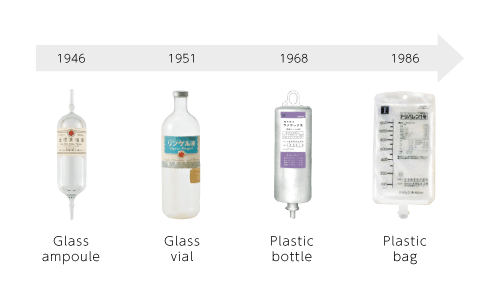
In 1946 when we began manufacturing various injectable solutions, the container was only made of glass. In order to overcome the disadvantage of a glass container which is heavy and easy to break, we developed plastic bottles for the first time in Japan, and in addition, we have offered the high-performance soft bags which improved the transparency and strength of containers, infection prevention measures, and convenience. Putting quality first, we are committed to materials and raw materials of containers, and we are developing our own containers with our strengths. We will continue to aim for container development that is even more innovative and can contribute to the medical field.
Utilizing the technology we have built up in the field of drug development, we have recently begun R&D in the field of medical foods, a growth area in both medical treatment and nursing. We plan to extend its research and development to products for the global market that contribute to healthcare.
In the event of dehydration caused by diarrhea, vomiting, or fever associated with infectious enteritis or the common cold, or due to excessive sweating or a lack of oral hydration in the elderly, it is essential to quickly replenish water and electrolytes. Based on the approach to oral rehydration therapy proposed by the WHO, we developed oral rehydration solution, OS-1, featuring a balance of electrolytes and carbohydrates. In December 2004, for the first time in Japan, OS-1 has been approved as a food for persons with medical conditions (individually evaluated type), categorized as a food for special dietary use by the Ministry of Health, Labour and Welfare. It is suitable for mild to moderate dehydration and is used broadly in clinical and nursing care settings.

Dietary intake involves sequential processes ranging from chewing, the formation of food mass, and swallowing. PROCESS LEAD, a chew- and swallow-managing food, has been developed to have a solid texture when eaten, yet becomes an easily swallowed paste after being chewed. It is a food that meets the needs of medical and nursing care settings and is designed to be eaten by people with reduced chewing and swallowing functions too.
