TOP > News Release > Otsuka Pharmaceutical Factory launches "Tumguide Fiber" with an additional specification of outer diameters of 0.5 mm and 0.75 mm to be used with "Tumguide," a device for checking the position of the nasogastric tube tip
November 8, 2024
Otsuka Pharmaceutical Factory, Inc. (Head Office: Naruto, Tokushima, Japan; President and Representative Director: Shuichi Takagi; hereinafter, "Otsuka Pharmaceutical Factory") will launch another "TumguideⓇ Fiber," a general medical device (gastroesophageal sterile tube and catheter for temporary use) with an additional specification of outer diameters of 0.5 mm and 0.75 mm (hereinafter, "this product") on November 14; this device is to be connected and used with "TumguideⓇ LED Light Source," a device for checking the position of the nasogastric tube tip.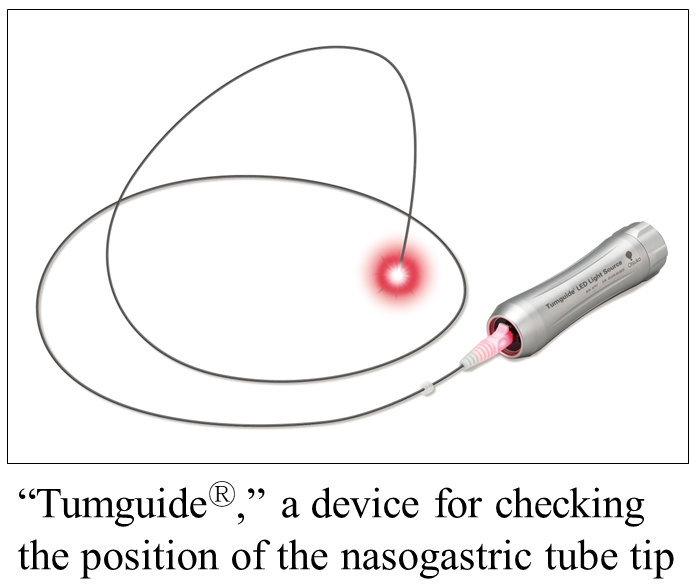
This product is an optical fiber used in pediatric treatment that can be inserted into the nasogastric tube. The fiber is inserted into the nasogastric tube, connected with Tumguide LED Light Source (a general medical device and general-purpose light source; sold separately), and inserted into the stomach with the light at the tube tip shining; this medical device can thus facilitate a visual check of the position of the nasogastric tube tip from outside the body by using the red light (biological transparency*1).
In addition to enteral nutrition and medication, nasogastric tube procedure, wherein a feeding tube is inserted through the nose into the stomach to reduce the pressure in the stomach, is used as an administration route in many cases of nutritional management of patients who have difficulty consuming an oral diet. However, if the nasogastric tube tip is shorter than the appropriate size, or if the tube is placed in an incorrect position prior to enteral nutrition/drug administration, it may cause life-threatening complications. When a nasogastric tube is positioned, the tube tip position is checked in various ways including X-rays, aspiration of gastric juice, confirmation of the pH of aspirated liquid, and auscultation of air bubble sound. This product can be inserted into the nasogastric tube used in pediatric treatment. We hope it will contribute to reducing the physical burden on patients and to reducing the workload of healthcare professionals by reducing risks in nasogastric tube placement in pediatric treatment and by helping to avoid radiation exposure.
As a company with strength in clinical nutrition such as IV solution and enteral nutrition products. Otsuka Pharmaceutical Factory has been providing various products and useful information for appropriate nutritional management. We will continuously provide these and develop innovative products to contribute to solving issues in medical fields, aiming to be the best partner for patients and healthcare professionals in the clinical nutrition field.
Based on the corporate philosophy of "Otsuka-people creating new products for better health worldwide," the Otsuka Group is dedicated to contributing to the health of people around the world.
*1 A light in the wavelength range which transmits through soft tissues, but not for hard tissues such as bones and cartilages.
Tumguide® Fiber (Outer diameter 0.5mm,0.75mm)
|
Medical device classification |
General medical device |
|
Generic name |
Gastroesophageal sterile tube and catheter for temporary use |
|
Medical device class category |
Class I |
|
Medical device notification number |
47B2X10003000002 |
|
Shape, structure and principle |
<Shape, structure>
<Principle> |
|
Purpose of use or effect |
Insert this product with the nasogastric tube into esophagus and stomach temporarily. By connecting this product to the light source device, the tip position can be visually checked from outside the body. |
|
Directions for use |
Refer to the instruction manual of the Tumguide LED Light Source for detailed directions of use. ① Take out this product aseptically. |
|
Storage and expiration date |
<Storage> <Expiration date> As stated on the product (By self-certification) |
|
packaging |
10 fibers per box |
|
Suggested retail price (excluding tax)*2 |
JPY 30,000 |
|
Manufactured and distributed by |
Otsuka Clinical Solutions, Inc. |
|
Marketed by |
Otsuka Pharmaceutical Factory, Inc |
|
Comarketed by |
Otsuka Pharmaceutical Co., Ltd. |
*2 The price is shown as a reference price.