TOP > News Release > Otsuka Pharmaceutical Factory launches Actreen, an intermittent urological catheter
December 20, 2021
Otsuka Pharmaceutical Factory, Inc. (Head Office: Naruto, Tokushima, Japan; President and Representative Director: Shinichi Ogasawara; "OPF") will launch an intermittent urological catheter "Actreen," a medical device, on January 26, 2022, for which OPF has concluded an exclusive marketing agreement with B. Braun Aesculap Japan Co., Ltd. (Head Office: Bunkyo-ku, Tokyo, Japan; President and Representative Director: Takeshi Fujiwara; "BBAJ").
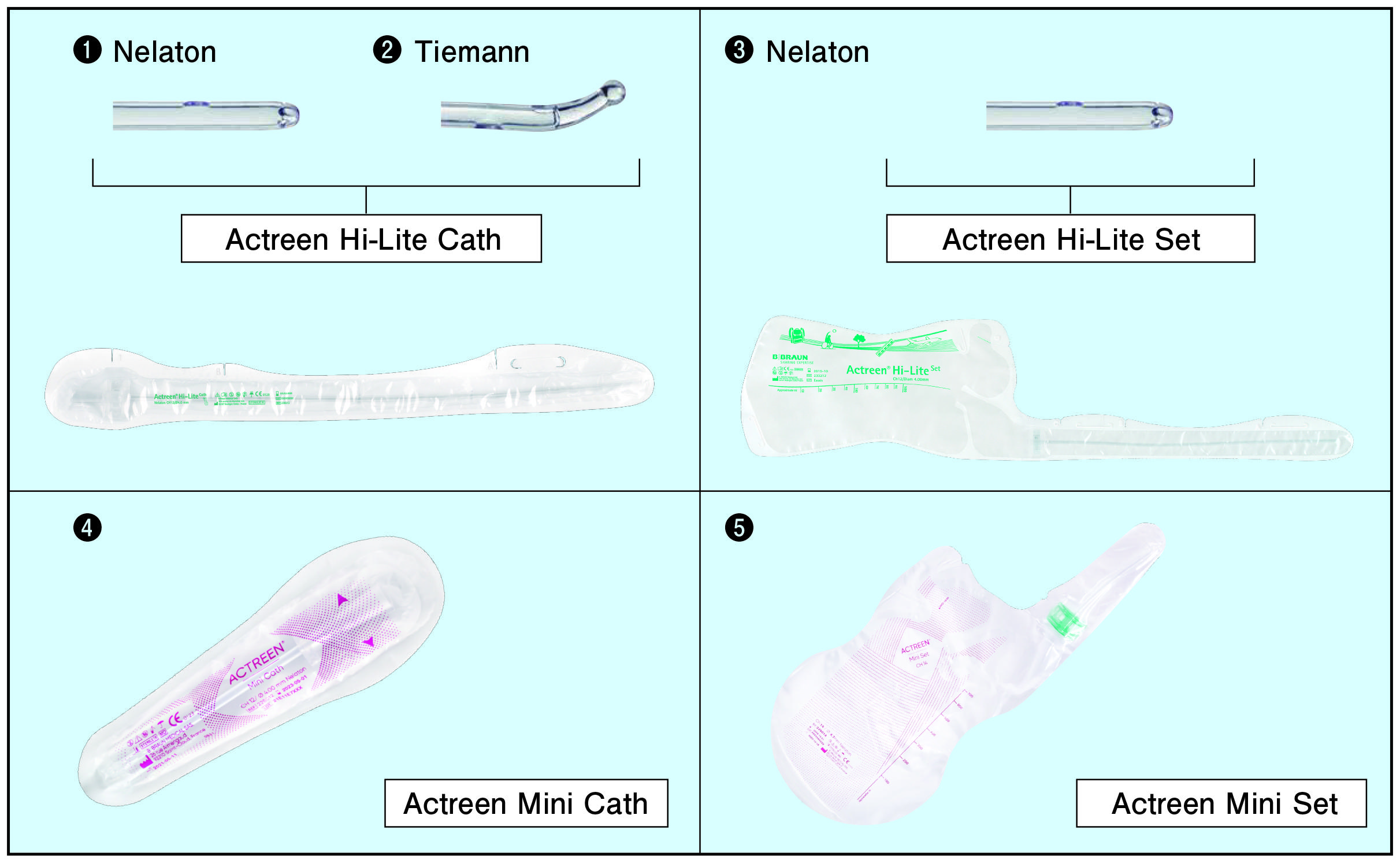
This product is a single-use catheter consisting of a catheter (tube) and connector that can be used during self-catheterization in people who have lost urge to urinate or who usually have difficulty urinating. The surface of the catheter is coated with a hydrophilic lubricant, which can be used immediately after opening, and also contains a product with an integrated urine collection bag that can be used according to the patient's condition. In this way, the product is designed with consideration for ease of opening and handling.
In the nursing care field in Japan, which has entered a super-aging society, the importance of excretion independence has been recognized and the need for excretory care is increasing, as evidenced by the review of the additional fee for excretion support in the 2021 revision of nursing care fees. In the event of residual urine or obstruction due to lower urinary tract obstruction, such as neurogenic bladder or prostatic hyperplasia, a method of clean intermittent drainage is widely used in which urine accumulated in the urinary bladder is catheterized through the urethral meatus at regular intervals to drain it out of the body, and a single-use hydrophilic product that is easy to use outside the hospital, such as at home care, has been desired.
BBAJ was established in 1986 as a Japanese subsidiary of B. Braun, a leading global healthcare company headquartered in Germany. With the vision of protecting and improving the well-being of people in Japan and around the world, they are contributing to Japanese medical care by delivering solutions covering diverse medical fields of B. Braun to Japanese healthcare settings.
The management vision of OPF is to be "The Best Partner in Clinical Nutrition." In addition, we are expanding our business areas to cover the entire healthcare process, such as prevention, diagnosis, treatment, and monitoring. Among these, we have been enhancing our products in the fields of urology and urination, including the marketing of Lilium® α-200,* a medical device that measures urinary bladder volume. We will continue to develop products that can contribute to excretion care for patients.
Based on the corporate philosophy of "Otsuka-people creating new products for better health worldwide," the Otsuka Group is dedicated to contributing to the health of people around the world.
*Bladder volume ultrasound imaging device developed and sold by Lilium Otsuka, a subsidiary of OPF, since November 2015 (Medical device approval number: 227ADBZX00146000)
| Product name | Actreen |
| Classification | Instrument & apparatus 51, Suckers, tubes and catheters for infusion or drainage |
| Generic name | Intermittent urological catheter |
| Medical device classification | Controlled medical device |
| Classification | Class II |
| Purpose of use or effect | The product is inserted into the bladder via the urethra and used for urinary drainage and urine collection. |
| Packaging | 30 units |
| Storage | Store away from heat, moisture, direct sunlight, and water. |
|
Types |
Catheter length (mm) |
Tip shape |
Outer diameter (CH) |
|||||
| 8 | 10 | 12 | 14 | 16 | ||||
|
❶ |
Actreen Hi-Lite Cath Nelaton |
367 |
2 holes Straight |
〇 |
〇 |
〇 |
〇 |
〇 |
|
❷ |
Actreen Hi-Lite Cath Tiemann |
367 |
2 holes Curved |
〇 |
〇 |
〇 |
〇 |
|
|
❸ |
Actreen Hi-Lite Set Nelaton |
367 |
2 holes Straight |
〇 |
〇 |
〇 |
〇 |
|
|
❹ |
Actreen Mini Cath |
90 |
2 holes Straight |
〇 |
〇 |
〇 |
||
|
❺ |
Actreen Mini Set |
90 |
2 holes Straight |
〇 |
〇 |
〇 |
||
 |