TOP > Our Business/Japan and Overseas > Quality Management

As a pharmaceutical company involved with human life, we place top priority on ensuring that everyone including patients and healthcare professionals can use our products safely. Quality is placed first at every stage from product R&D to manufacturing and distribution.
As a matter of course we comply with all laws, government regulations, and industry standards and have put in place a rigorous quality management system that takes into account the characteristics of each product.
We ensure that everyone working in the areas of manufacturing and quality assurance feels a strong sense that they play an important role in providing medical treatment. With this sense of responsibility, we work on thorough quality management to stably deliver safe, high-quality products.
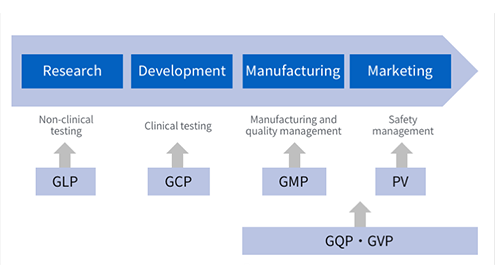
In pharmaceutical R&D, we comply with regulations (GLP, GCP) regarding clinical and non-clinical testing, and demonstrate not only the efficacy of candidate pharmaceutical substances but their guaranteed safety. We also strive to improve reliability through proper data management. We aim to build and implement a GMP system for manufacturing that will allow us to maintain the high quality of our products and consistently improve on that quality. From manufacturing through to marketing, based on GQP and GVP, we implement quality management and post-marketing safety management, and prepare and update reports and documentation that we submit to the regulating authorities. We also practice pharmacovigilance, i.e., safety monitoring that involves constantly collecting and evaluating product safety data, the results of which we promptly share with medical organizations and distributors.
Around the world today, strict standards are being rapidly established with regard to the quality and safety of pharmaceuticals, and there are especially strong calls for rigorous quality control of IV solutions, as products which are injected directly into the body. As the leading manufacturer of IV solutions in Japan, and being in the position of providing technical cooperation for and managing the IV business of the Otsuka Group that is active internationally, we have established and proactively operate a robust global quality assurance system. A framework for exchanging safety data has been established for the 15 group companies engaged in our IV business across 10 countries. In addition to complying with the laws and government regulations of each country, and industry standards, we are working as one to unify our approach to quality assurance and steadily maintain and improve product quality.

Global Quality Assurance Officers' Meeting
At Otsuka Pharmaceutical Factory, a dedicated department corresponding to the nature of the inquiry handles inquiries from patients, their families, medical personnel, and other stakeholders. We take great care to listen sincerely to people's perspectives, and strive to provide answers in a polite, honest, easy-to-understand way. We promptly give the information we acquire on safety and quality from these calls to the pertinent department. We also provide information to callers on monitoring risks and proper use, while using the feedback they give us to improve and develop products.

Intravenous Drug Information Center operator